
SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
To sketch the SO3 Lewis structure by following these instructions: Step-1: SO3 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SO3 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for SO3 generated from step-1 and step-2.

Image Showing Resonance Strcuture Of So3 So3 Resonance Structures Clipart Full Size Clipart
On the other hand for sulfurous acid, H 2SO3, we have 4 × 6 + 2 = 13 ⋅ valence electron pairs to distribute.. And thus O = .. S( − OH)2.the central sulfur is sp3 − hybridized, and the electron pairs assume a tetrahedral geometry. But molecular geometry is described in terms of ATOMS not electron pairs.and so the geometry around.

Draw The Lewis Dot Structure For So3 2 slidesharedocs
What is the molecular geometry of SO3 ^-2 a. draw its Lewis structure b. state its numeric code c. state its molecular geometry d. how many lone pairs of electrons are present on the central atom in the Lewis structure of sulfite ion? e.

So3 Lewis Structure 2 JalentuGentry
The Lewis structure for SO 32- is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 3 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.

Draw The Lewis Structure Of So3 2 Fotodtp
Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair while all the three Oxygen atoms have 2 lone pairs.

So3 Lewis Structure With Formal Charges
Hello Guys!The sulfite ion comprises one Sulfur Atom and three Oxygen atoms. The ion has a negative charge as it accepts two additional electrons. The video.

SO3 Lewis Structure Sulfur Trioxide YouTube
The Lewis structure of sulfite [SO3]2- ion is made up of a sulfur (S) atom and three oxygen (O) atoms. The sulfur (S) is present at the center of the molecular ion while oxygen (O) occupies the terminals, one on each side. There are a total of 4 electron density regions around the central S atom in the Lewis structure of [SO3]2-.

Chemistry Worksheets, Chemistry Notes, Lewis, Dots, Activities, Development, Structures, Stitches
Draw the Lewis structure for the sulfite ion, SO3 2−. Which of the statements below is true for the Lewis structure of the sulfite ion? a)There are double bonds between the sulfur atom and each of the three oxygen atoms. b)There must be a double bond between the sulfur atom and one of the oxygen atoms to ensure that all atoms have an octet.
Resonance Structures So2 So3 No2 So3 2 Nitrite
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO 3. It has been described as "unquestionably the most important economically" sulfur oxide. [1] It is prepared on an industrial scale as a precursor to sulfuric acid .

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Lewis Dot Structure of SO3 2- (Sulfite Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 196K views 12 years ago Every Video I quickly take you through how to draw the Lewis.

SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2 (Sulfite Ion) YouTube
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure of SO3
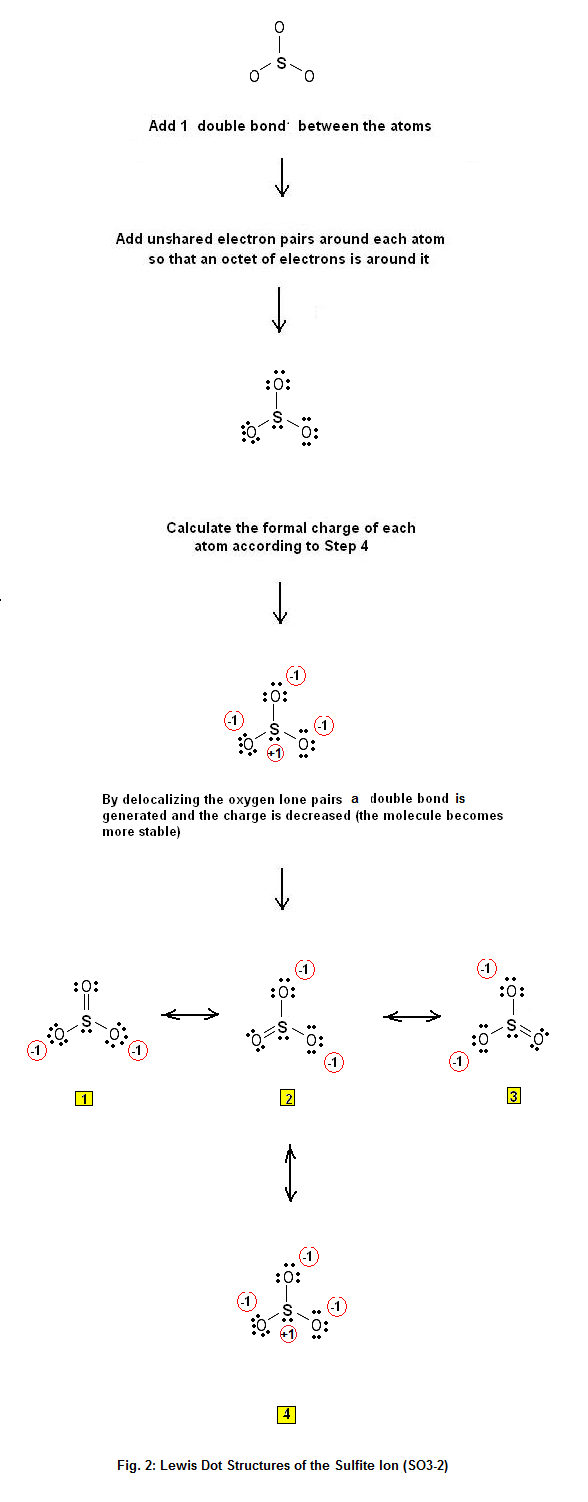
Lewis Dot Structure of the sulfite ion SO32 Electron Dot Structure Advanced Chemistry
Wayne Breslyn 724K subscribers Join Subscribe Subscribed 1.9K Share 411K views 10 years ago A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3.

Lewis Dot Structure For So3 slidesharedocs
Following steps are required to draw the SO 32- lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of sulfur and oxygen atoms Total electrons pairs Center atom selection Put lone pairs on atoms Check the stability and minimize charges on atoms by converting lone pairs to bonds.

Resonating structure of SO3 Chemistry Q&A
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry of sulfite ion (SO3 2-).

michiganswebdesigners Sif6 2 Lewis Structure
SO3, known as sulphur trioxide is sp2 hybridized with a triagonal planar structure and having bond angle 1200. It is a colourless or white crystalline solid with boiling and melting point 450C and 16.90C respectively. It is a covalent compound having total three double bonds in between sulphur and oxygen are present in SO3 structure.